Existing standalone ventilators rely on the global supply chain to source hundreds of subcomponents. The demand for ventilators is increasing rapidly worldwide due to the COVID-19 illness. However, many countries, including Canada, have stopped exporting medical equipment, resulting in equipment shortages. This resulted in our client, Toronto General Hospital (TGH), in need of an open-source ventilator that is invasive and a Continuous Mandatory Ventilation (CMV) that incorporates a Pressure Regulated Volume Control (PRVC) mode that reduces the risk of causing barotrauma to the patient.
The design requirements were the following:
- Must be able to transport breathable air into and out of the human lungs by extracting air from an air and/or oxygen source, sense the state of the patient, and mechanically transport air into and out of the lungs.
- Must adhere to key constraints set by the Medicine & Healthcare Products Regulatory Agency (MHRA), such as ventilator control parameters (flow rate, I:E ratio, Peak Inspiratory Pressure, and Plateau Pressure) and the capability of continuous operation for up to 14 days only in a non Intensive Care Unit (ICU).
- Satisfy key objectives set by MHRA, such as FiO2, Tidal Volume, Respiratory Rate, Positive End Expiratory Pressure (PEEP) parameters and the implementation of monitoring and alarm systems.
- Should use license-free hardware, software, and components that can be easily accessed around the world and be rapidly manufacturable.
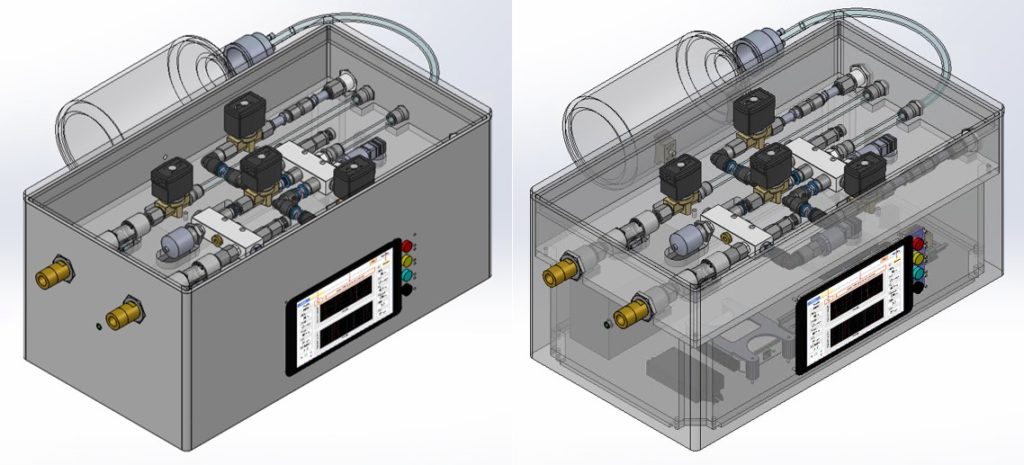
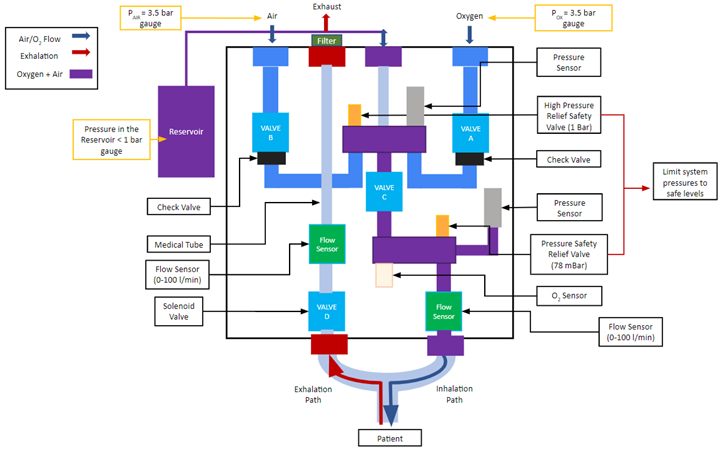
The design is composed of two main systems: pneumatic system and electronic/hardware system. The pneumatic system consists of the following main components shown in Figure 2: Valve A (1), Valve B (2), Valve C (3), Valve D (4), 2L bottle reservoir (5), pressure sensors (6), pressure relief valves (7), oxygen sensor (8), and flow sensors (9). The electronic system incorporates the following main electrical components: uninterruptible power supply, Logic Module, DAQ, and embedded computer (Raspberry Pi).
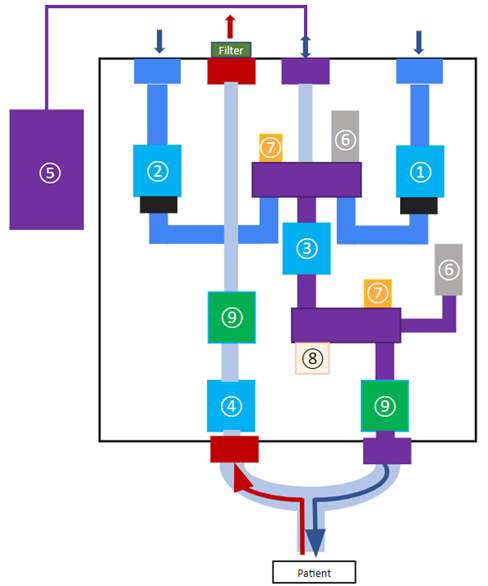
The design employs three main phases per respiratory cycle during ventilation: inhalation, reservoir filling, and exhalation. Valves A, B, C, and D are direct-acting solenoid valves that govern these phases. This is done by regulating gas flow rates based on pressure drops across each valve established by their flow factor (KV). The higher the flow factor number a valve is rated at, the better the efficiency of a fluid flow through the valve. Valves A and B control the air and oxygen supply to the reservoir. Valve C leads the gas mixture from the reservoir to the patient during inhalation. Valve D leads the inhaled gas in the patient’s lung to the atmosphere. Valves A, B, and C are normally closed and Valve D is normally open. This ensures that if the power to the ventilator were to be cut during ventilation, all gas flow from the ventilator is cut and air can enter and exit from the patient through Valve D to the outside.
For each phase, their respective design challenges were mainly solved analytically and mathematically utilizing fluid mechanics principles, such as Reynolds Transport Theorem, pressure drop across valves, ideal gas law, and subcritical and supercritical flows. A process simulator called Aspen HYSYS was then utilized to simulate our analytically derived solutions for each phase. But because this software is mainly used for chemical plant design, the program is unable to simulate lung properties. It does however provide us with a general idea of flow rates and pressure changes across the system which we can use to compare our mathematically derived calculations.
The design of this open-source ventilator was created with the generous support from our supervisor, Professor Behdinan, our client, Toronto General Hospital, and the global community that has sustained a continuous effort in producing open-source ventilators to combat ventilator shortages in hospitals. The two notable open-source ventilator designs we have gained a tremendous amount of invaluable information and resources include JAMVENT by Imperial College London and Makair. We would like to share our deepest gratitude towards the teams behind these designs as our design would not have been conceivable without their support.
Unfortunately due to COVID-19 and the restrictions that have brought with it during the academic year, we had limited access to labs and cleanrooms where we could build, program, and test our design. The validation of the design, notably the gas flow characteristics, was exclusively performed on a process simulator. Physical testing using a test lung and animals must be performed to assess the safety and efficacy of the ventilator under the supervision of certified clinicians and doctors.